Controlling the Cold Chain: How to Monitor Vaccine Temperatures from Start to End

Whether a vaccine is in development, in transit, or in storage - continuously monitoring the temperature and environmental conditions of the product is a crucial step in preserving and maintaining its efficacy. According to the CDC, failure to store and handle vaccines properly can reduce potency, which results in inadequate immune responses in patients and poor protection against diseases. This, ultimately, deems the vaccine ineffective. Whether you’re a drug manufacturer, distributor, or provider - it’s a shared responsibility among all involved to monitor and maintain proper environmental conditions and ensure vaccine efficacy for patients and maintain CDC/VFC compliance.
What is the Vaccine Cold Chain?
The vaccine cold chain simply describes every step of the vaccine supply chain, each requiring a temperature-controlled environment. The cold chain begins with the cold storage unit at the manufacturing plant, followed by the transport and delivery of the vaccine, and ends with proper storage at the provider facility prior to administration of the vaccine to the patient.
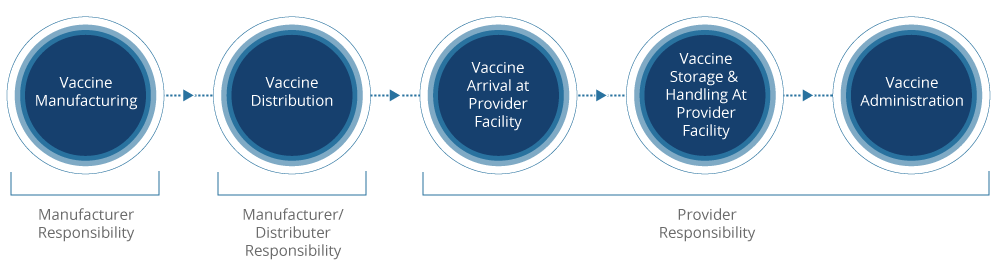
Improper storage of vaccines can lead to unwanted conditions, including overexposure to heat, cold, or light at any step in this cold chain. Ultimately, this results in a complete loss of vaccine usage. In fact, the CDC says that exposure to any improper conditions can affect the potency of a refrigerated vaccine, but a single exposure to freezing temperatures can actually destroy potency. One mishap in the cold chain can mean extra doses for patients, increased costs for providers, and mistrust from the public about vaccine confidence.
CDC/ VFC Compliance & Storage
Vaccine providers, specifically health clinics and primary care providers, are usually bound to the compliance requirements set forth by the CDC’s program, Vaccines for Children (VFC). This is a federally funded program that provides vaccines to children who might not otherwise be able to get vaccinated because of associated costs. The CDC buys these vaccines at a discount, distributes them to grantees (usually state health departments), who then distributes them at no charge to registered VFC providers. In order to become a VFC provider and receive these vaccines, organizations must maintain VFC compliance and keep up with CDC recommendations for vaccine handling and storage.
The CDC provides an exhaustive list about proper vaccine storage, vaccine inventory management, vaccine preparation, and vaccine transport. Beginning with the drug’s manufacturing and ending with administering the vaccine to patients, there are a few key items to implement into your cold chain to ensure proper storage of vaccines and maintain VFC compliance:
- Use purpose-built or pharmaceutical-grade refrigerator/freezer units.
- Use a temperature monitoring device (TMD) that utilizes buffered temperature probes, the most accurate method of measuring vaccine temperatures.
- Keep storage data for three years to analyze long-term trends and/or recurring problems.
- Ensure proper calibration of TMD sensors, including date of recalibration, serial numbers, and device numbers.
- Have a proactive plan ready for power outages and network disruptions.
- Find a TMD with the following features:
- Alarming system for out-of-range temperatures.
- Current, minimum, and maximum temperature display that can be easily read from outside the unit.
- Recommended uncertainty of +/-0.5° C (+/-1° F).
- Logging interval to measure and record temperatures at least every 30 minutes.
Sonicu Vaccine Monitoring
Maintaining compliance can be a labor intensive and time consuming process if not automated by a system designed to eliminate these challenges. Sonicu provides vaccine monitoring kits for every step of the cold chain. Our monitoring system was designed to help vaccine providers meet and exceed the requirements of the CDC, VFC program, and other regulatory agencies. By automating vaccine monitoring, organizations not only ensure vaccine efficacy and safety, but they unburden already busy staff from the manual processes of maintaining vaccine compliance.
Sonicu generates audit-ready compliance reports, manages the calibration of sensors, and makes ongoing vaccine monitoring easy. With our Implementation Wizard, setup and installation of a Sonicu monitoring system can be done by anyone - regardless of technical expertise - and within minutes! Here are some features of a Sonicu vaccine monitoring system:
- Automatically monitors and logs all sensor data 24/7
- Battery backup and data sync ensure no data is lost if a power outage or network interruption occurs
- Alert notifications sent via phone, text, or email if temperature, network, or power loss occurs
- 24/7 access to live data via Sonicu mobile app or SoniCloud desktop
- Wireless hardware can be installed anywhere and connected via WiFi, cellular, or RF options without the need for IT support or building modifications
- Automatically generates audit-ready compliance reports
- Secure, cloud-based platform hosted on AWS
- Ongoing NIST-certified calibration program for sensors
- Wireless display can be installed on the outside of refrigerator/freezer units
Sonicu is used and approved by VFC providers from coast to coast. If you think your organization could benefit from a Sonicu vaccine monitoring kit, contact us below to learn more.