Clean Room Monitoring Regulatory Standards
If you are looking for Clean Room Monitoring Regulatory Standards that help you and your team eliminate manual logging, improve compliance readiness, and protect all your temperature-sensitive assets, you’ve arrived at the right place.

Let us help you evaluate your needs!
- Safety: Alerts via text, email, push notifications and phone calls to protect your precious assets
- Compliance: Automated compliance reports
- Efficiency: Reduced Manual Logging and time spent on reports
And what makes us different?
- Lifetime Warranty: Never buy hardware again!
- Unlimited Users: Scale across your entire organization
- Connectivity Flexibility: Wi-Fi, Cellular or Data Hub
- Phone call alarms: Alerts won't get ignored
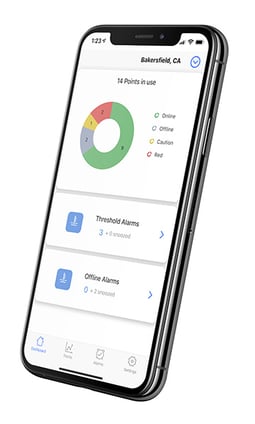
- Mobile App: 500 Freezers in your pocket
- Facility monitoring: Simple to add water leak, door open, occupancy, and even IAQ monitoring
Engineered in Indiana with U.S.-based support.
See What Customers Say About Sonicu
Asset Protection. Compliance Automation. And Reduced Manual Processes.
Sonicu serves thousands of professionals at hundreds of organizations across North America by improving how they monitor and manage their most sensitive assets and environments.
Professionals from healthcare, life science, laboratory and cold chain facility management turn to Sonicu to help them improve the way they do business.

How IU Health
consolidated all of its pharmacy monitoring needs
into one cloud-based platform serving dozen of locations.


Clean Room Monitoring Regulatory Standards
Some of the finest names in healthcare, including Indiana University Health, the University of Michigan Health System, and Stanford University, rely on Sonicu to provide a robust and continuous environmental monitoring system, including temperature, humidity, air pressure, and more.
These respected healthcare and research brands turn to Sonicu for four primary reasons:
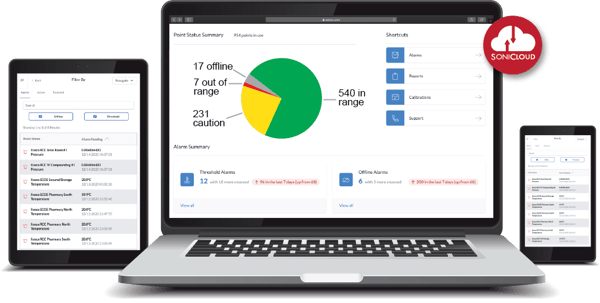
- Real-Time Monitoring: the sensors collect temperature, humidity, air pressure data and more, and transmit it wirelessly to SoniCloud - our cloud-based platform.
- Operational Efficiency: Virtually eliminate the need for tedious and costly manual logging
- Compliance Automation: Respond to any regulatory audit or inspection in a few clicks with our reports section
- Asset Protection: Detect and respond to any threshold that can threaten anything perishable: food, drugs, vaccines, research, etc.
Our customers stay with us thanks to our American-based customer support, which is never more than a phone call away. While our technology is intuitive and powerful, we know it’s only as strong as the people who stand behind it.
Deeply enmeshed in a network of rules and guidelines, clean room monitoring regulatory standards are paramount to maintaining the integrity of several industries.
Maintaining a delicate equilibrium could be the determining factor between achievement and setback.
Monitoring cleanrooms isn’t just routine; it's a carefully regulated process, like a well-coordinated dance following strict standards.
These standards outline everything from frequency of monitoring to particle size parameters.
Ranging from ISO cleanroom standards which provide a globally recognized framework, to more location-specific guidelines, all are designed with one goal in mind: ensuring an environment of pristine sterility.
Clean rooms are classified into different grades: A, B, C, and D. Each grade requires specific monitoring guidelines to ensure seamless operations.
A Grade A room, for instance, is the most strictly controlled, while a Grade D room allows for more variability in microbial and particulate counts. Sonicu customers,required to navigate these classifications, streamlines the process of creating, managing, and maintaining compliant clean rooms.
Every clean room standard is put into place to assure proper monitoring, which plays an instrumental role–especially in the pharmaceutical industry.
These meticulous procedures and standards might seem overwhelming, but organizations like Sonicu aid in maintaining the stringent guidelines that encompass clean room monitoring:
- Sonicu affords automated solutions which offer seamless integration with existing systems
- real-time alerts, enabling immediate action to any deviations from standards
- Data logging capabilities offered by Sonicu further aid clean room compliance by providing requisite information for regulatory purposes
- Sonicu's smart sensors and cloud-based platform simplify the complexity and help to proceed with confidence in maintaining clean room standards.
Ultimately, Sonicu enables companies to concentrate on their operations, offering assurance regarding regulatory standards and cleanroom monitoring. The interdependent nature of these guidelines and Sonicu underscores their crucial role in maintaining the integrity of highly sterile environments.
You can learn more about how our software helps compliance professionals in these case studies:
Problem: Needed to reduce time to complete HACCP compliance reports
Solution: Self-install of temperature monitoring for compliance automation
Problem: Needed pharmacy temperature monitoring following refrigerator loss across many remote clinics:
Solution: Simple Sonicu installation and configuration simplified standing-up remote systems
Indiana University Health: Enterprise Hospital For Temp, Humidity, Air Pressure
Problem: Server Based Monitoring lacking Enterprise Visibility
Solution: One Window into all monitoring across dozens of facilities
Understanding Clean Room Classifications
Understanding clean room classifications requires a keen comprehension of the regulatory standards that guide clean room monitoring. These are meticulous guidelines, set by industry authorities, to ensure that controlled environments are kept under the most stringent cleanliness conditions.
What exactly qualifies as a clean room, one might ask?
The answer lies in the clean room classification chart. This tool is a systematic guideline that categorizes clean rooms based on the permitted level of contaminants. Contaminants, under these criteria, can range from microscopic particles to various airborne microbes.
Digging even deeper, we find clean room class 100. This classification signifies a room where there are no more than 100 particles, sized 0.5 micron or larger, in one cubic foot of air. This rigorous standard is often required in industries such as pharmaceutical manufacturing or biotech, where the utmost level of cleanliness is paramount to prevent product contamination and ensure the safety and efficacy of the end product.
This brings us to the realm of ISO clean rooms.
The International Organization for Standardization delineates several classes of clean rooms, from ISO 1 to ISO 9, each with its unique specifications. An ISO 1 clean room exhibits the utmost purity, allowing the smallest concentration of airborne particles.
In contrast, ISO 8 and ISO 9 clean room requirements are less rigorous, but not any less essential in their respective industries. ISO Class 8 and Class 9 clean rooms have more liberal guidelines regarding the amount and size of permissible particles per cubic meter. However, they still maintain rigorous standards on airflow, pressure, temperature, and humidity.
How does all this information relate to Sonicu?
- Sonicu's range of wireless monitoring systems is an ideal companion for clean rooms of every classification.
- The state-of-the-art technology deployed by Sonicu offers accurate, real-time monitoring of critical parameters such as temperature and humidity, which are vital for keeping clean rooms within specified cleanroom classifications.
- Serving a diverse array of industries such as healthcare, food safetypharmaceuticals, and biotech, Sonicu’s solutions help businesses meet compliance with rigorous cleanroom standards, maintaining utmost product quality and safety.
- Sonicu's robust data logging and alerting features allow for immediate corrective action when deviations from set parameters occur, thus helping to maintain and monitor the integrity of clean rooms.
Understanding clean room classifications and monitoring requirements is crucial to maintaining cleanliness standards and ensuring product and process integrity.
Sonicu solutions makes it easier than ever for businesses to meet and exceed those standards.
Here are some case studies that highlight the solutions offered by Sonicu:
- Ohio University: https://www.sonicu.com/ohio-university-innovation-center-temperature-and-environmental-monitoring
- Katherine Shaw Bethea: https://www.sonicu.com/katherine-shaw-bethea-hospital-pharmacy-temperature-monitoring
- CleanSlate Addiction Treatment centers: https://www.sonicu.com/cleanslate-treatment-center-temperature-monitoring
Regulatory Standards In Clean Room Monitoring
Comprehensive knowledge and adherence to regulatory standards in clean room monitoring are essential elements of integrity in numerous scientific and industrial sectors, including pharmaceuticals, biotech, and electronics. Guided by the FDA and other authoritative bodies, these standards dictate the parameters of cleanliness, ensuring the maintenance of a steadfast sterility in these critical environments.
Regulatory standards play a crucial role not just in clean room monitoring, but also in other associated measures such as clean room air changes. Air changes are routinely regulated under the standards to circulate and cleanse the air, preventing the accumulation of particulates that could jeopardize the sterility of the room.
This control measure, alongside others, reflects the multifaceted nature of clean room regulatory standards, emphasizing the broader objective to maintain airtight cleanliness
The stringent clean room monitoring regulatory standards underpin the integrity of products developed and manufactured in these environments. Adherence to these precise standards, monitored and enforced by authoritative bodies, ensures that industries produce safe and reliable products.
Regulatory standards not only delineate the parameters of cleanliness but also provide guidelines for corrective and preventative measures in case of deviations.
The imperative of understanding and abiding by U.S. Food and Drug Administration (FDA) clean room standards cannot be overstated. Defined by the FDA, these standards provide a regulatory framework for clean room management. They address clean room classification, environmental monitoring, and control measures needed to guarantee sterility.
- Sonicu's innovative environmental monitoring solutions are designed to ensure compliance with these standards, real-time data logging and reporting to ensure continuous clean room sterility.
- Sonicu’s wireless monitoring systems for clean room air changes align with regulatory standards, providing accurate air change rates data and helping to ensure ongoing compliance.
- Sonicu's platform follows clean room monitoring regulatory standards, securing a highly controlled environment that promotes safety and quality.
- Sonicu's solutions align with FDA clean room standards, providing industries with a reliable tool to sustain clean room sterility and comply with regulatory measures.
Guidelines For Pharmaceutical Clean Rooms
Pharmaceutical clean rooms are a cornerstone of the industry, dictated by stringent guidelines that ensure the production area remains uncontaminated and sterile.
These protocols adhere to the "Good Manufacturing Practice" (GMP) and are essential in guaranteeing product quality, reliability and safety for consumers. The elements covered in the "Guidelines for pharmaceutical clean rooms" encompass a range of procedures, including environmental monitoring, the use of appropriate clothing, and regular sanitization practices.
A key component in these guidelines is the clean room classification. This is a system that separates clean rooms based on the level of cleanliness to create a controlled environment supportive of a specific production process.
Sonicu's wireless temperature and humidity monitoring systems act as effective tools in maintaining the required environmental conditions for a particular clean room classification, thereby ensuring compliance with industry standards.
Sonicu, capable of integration into existing Building Management Systems, supports clean room standards by providing accurate, real-time data used to maintain a stable, clean environment.
Thus, these guidelines are critical in the pharmaceutical industry. They provide a safe and effective operational environment essential for maintaining product quality and end-user safety. Sonicu's innovative products play a vital role in supporting these rigorous standards and regulations. Understanding these guidelines and how Sonicu fits into their application aids in appreciating their importance in the pharmaceutical landscape.
You can learn more about how our software helps compliance professionals in these case studies:
Problem: Pharmacy Suffered Too Much Humidity In New Wing, impacting Compounding Pharmacy
Solution: Affordable Humidity Monitoring that Delivered Powerful data to prompt contractors to fix improperly sized air handler
Problem: The dining department struck with regulatory violations
Solution: Enterprise-wide monitoring that automates regulatory compliance across all departments
Problem: Release of lead particles in battery projects
Solution: Mobile, affordable air pressure monitoring solution
Importance Of Microbial Monitoring In Clean Rooms
The importance of microbial monitoring in clean rooms cannot be overstated. These aseptic environments are spaces where critical operations, such as surgery or vaccine production, are accomplished. Given the sensitive nature of these activities, ensuring the cleanliness and sterility of these rooms is paramount. A single microbe can create havoc and compromise the integrity of the entire process. This is where clean room microbial monitoring comes into play. It offers a proactive approach to maintaining the rigorous sanitation levels required in these spaces.
- Sonicu understands how vitally essential cleanliness and sterility are to these spaces and offers a range of monitoring solutions designed for clean room applications. Their products and services help ensure that clean rooms meet and, on many occasions, exceed established regulatory requirements.
Every country has its clean room monitoring regulatory standards. These regulations ensure that clean rooms effectively prevent, reduce, or eliminate the risk of microbial contamination. They dictate everything about clean rooms, from design and construction to operation and maintenance. They also mandate regular microbial monitoring to ensure ongoing compliance with these standards. Noncompliance with these regulations can lead to severe penalties, including fines or being shut down.
- Sonicu's compliance-monitoring products are designed to comply with the stringent regulations of clean room monitoring. An advanced cloud-based platform enables an automated, reliable, and easily accessible monitoring system, saving operational time while ensuring regulatory requirements are consistently met.
Microbial monitoring in clean rooms is therefore not just a good practice - it is a necessity. It provides an early warning system for potential contamination risks and allows quick, preventive actions to minimize these threats. Given the valuable work performed in clean rooms, investing in high-quality microbial monitoring systems is not an expenditure but an investment in safety and quality.
- Sonicu provides smart sound and environmental monitoring solutions, including temperature, humidity, and pressure – all vital in maintaining the strict conditions needed in a clean room. These monitoring systems support a swift response to potential contaminant risks, playing a critical role in maintaining the sterile conditions that clean rooms require.
To neglect microbial monitoring in clean rooms is to risk the virtues of sterility and precision that these unique spaces represent. Staying on top of this essential aspect of clean room operations is not just about maintaining the status quo but ensuring the best possible outcomes in whatever endeavor depends on these sterile environments.
- Sonicu's devotion to providing comprehensive monitoring solutions supports clean room operations' critical nature. Its products are testimony to the importance it places on advancing technologies to promote safety and efficiency in sterile environments.
Significance Of Portable Clean Rooms
The use of portable clean rooms can greatly contribute to the dynamic landscape of various high-tech industries. A clean room is a controlled environment where products are manufactured. It is a room in which the concentration of airborne particles is controlled to specified limits. This exactitude in maintaining containment levels makes the spaciousness of factories irrelevant, giving way to the significance of portable clean rooms.
The most significant advantage of portable clean rooms is in regards to their flexibility. Unlike standard clean rooms, portable versions can be easily transported and swiftly set up, saving valuable time and resources. They can be relocated to the desired area of operation, ensuring a clean environment wherever the industry demands.
Furthermore, these cubicles of cleanliness adhere to the stringent norms laid down by the ISO 7 Clean Room standards. These standards, also known as Class 10,000 Clean Rooms, dictate the acceptable levels of particulate matter in a specific volume of air, thereby catalyzing fabrication and manufacturing processes reliant on sterile conditions.
When considering the role of Sonicu in this context, the company’s wireless monitoring solutions are designed to ensure the seamless operation of these rooms. With smart sensors and remote monitoring, Sonicu solutions contribute to:
- Maintaining pre-determined cleanliness levels as per ISO 7 specifications.
- Enhancing the portability of clean rooms with their wireless sensors.
- Ensuring all-time monitoring that negates the need for human intervention.
Plus, Sonicu, with its monitoring solutions, can guarantee real-time information about the environmental conditions inside these clean rooms.
In conclusion
- Sonicu's remote monitoring facilitates easy, accurate tracking of conditions inside portable clean rooms.
- Their wireless solutions amplify the utility and flexibility of portable clean rooms.
- Sonicu’s sensors offer an additional layer of quality control, enhancing the standards set by ISO 7, and facilitating seamless operations in various industries.
Therefore, the significance of portable clean rooms cannot be overstated in industries where maintaining a sterile environment is not just a necessity but a mandate. Their conformance to international standards, coupled with the Sonicu industry-leading solutions, helps to ensure consistent quality and reliable control over contamination. The collaboration resulting from this can reap enormous technical and financial advantages.
American-based Customer Support: Robust & Reliable High Touch Service
Software and technology is only as good as the people who stand behind it.
At Sonicu, that means our team of American-based customer success managers who are never more than a phone call away to help field and fix any service issues.
Our probes and sensors are placed in demanding frozen environments and our software literally sends billions bits of data monthly, meaning there’s always the potential for a hiccup on either the hardware or software.
We are committed to fielding every customer service request promptly and addressing our customer’s concerns promptly and professionally.
 “I like to say that every refrigerator or freezer is like a car in that they all behave a bit differently,
“I like to say that every refrigerator or freezer is like a car in that they all behave a bit differently,
and then every now and then you just get a bad boy who doesn’t want to perform as we need it to,”
Martha Rardin, Director, Nutrition and Dietetics, Hendricks Regional Hospital.
 “Sonicu has been a powerful tool to identify which units are behaving out of spec and get our team
“Sonicu has been a powerful tool to identify which units are behaving out of spec and get our team
to fix them before we have a serious issue.”
Tim Livesay, Director, Hancock Regional Hospital Pharmacy Director






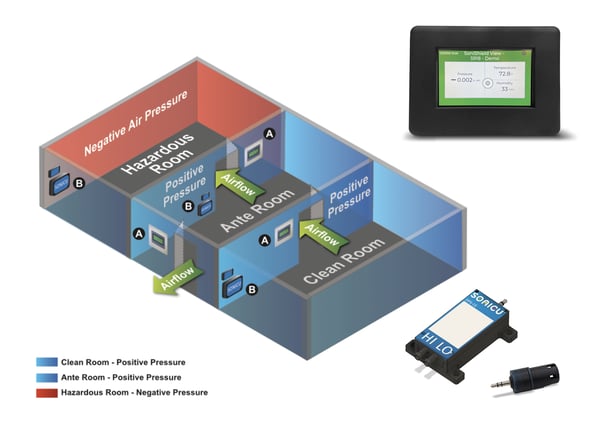


Before Sonicu, we had to don bunny suits to check the status of our cleanrooms. Now we check our phones and know right away. The system saves us time and effort and helps us respond to environmental