DSCSA 2023 Requirements
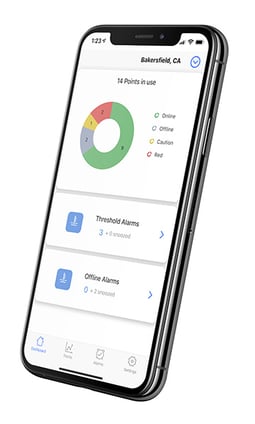
If you’re looking for a cloud-based hospital refrigerator (or freezer) temperature monitoring system that helps your team eliminate manual logging, improve compliance readiness and protect all your temperature sensitive assets, you’ve arrived at the right place.

Let us help you evaluate your needs!
- Safety: Alerts via text, email, push notifications and phone calls to protect your precious assets
- Compliance: Automated compliance reports
- Efficiency: Reduced Manual Logging and time spent on reports
And what makes us different?
- Lifetime Warranty: Never buy hardware again!
- Unlimited Users: Scale across your entire organization
- Connectivity Flexibility: Wi-Fi, Cellular or Data Hub
- Phone call alarms: Alerts won't get ignored
- Mobile App: 500 Freezers in your pocket
- Facility monitoring: Simple to add water leak, door open, occupancy, and even IAQ monitoring
Engineered in Indiana with U.S.-based support.
See What Customers Say About Sonicu
Asset Protection. Compliance Automation. And Reduced Manual Processes.
Sonicu serves thousands of professionals at hundreds of organizations across North America by improving how they monitor and manage their most sensitive assets and environments.
Professionals from healthcare, life science, laboratory and cold chain facility management turn to Sonicu to help them improve the way they do business.



DSCSA 2023 Requirements
If you’re looking for a cloud-based hospital refrigerator (or freezer) temperature monitoring system that helps your team eliminate manual logging, improve compliance readiness and protect all your temperature sensitive assets, you’ve arrived at the right place.
Some of the finest names in healthcare, including Indiana University Health, MiraVista Diagnostics and Piazza Produce (as well as University of Michigan Health System and Stanford University), rely on Sonicu to provide robust continuous temperature monitoring for their cold and frozen environments.
These respected healthcare and research brands turn to Sonicu for three primary reasons:
- Operational Efficiency: Virtually eliminate the need for tedious and costly manual logging
- Compliance Automation: Respond to virtually any regulatory audit or inspection in a few clicks with our reports section
- Asset Protection: Detect and respond to any temperature excursion that can threaten virtually anything perishable: food, drugs, vaccines, research, etc.
When you combine these three significant feature benefits, our clients average about $80,000 of savings for every 100 hospital beds.
At research or university locations without beds, it’s not hard to imagine the time savings alone by simply not having staff manually record temperatures several times per day.
Our customers stay with us thanks to our American-based customer support that is never more than a phone call away.
While our technology is intuitive and powerful, we know it’s only as strong as the people who stand behind it.
The Drug Supply Chain Security Act (DSCSA) is a legislative act that seeks to combat the harmful impact of counterfeit, stolen, or contaminated prescription drugs in the United States.
It establishes a range of requirements for healthcare organizations that are intended to improve package-level electronic tracing for certain prescription drugs and ensure interoperability between different partner organizations throughout the whole drug supply chain.
Among the most significant DSCSA 2023 requirements are standardized DSCSA serialization requirements.
The law also establishes specific DSCSA wholesale distributor requirements.
The Drug Supply Chain Security Act is a part of the Drug Quality and Security Act (DQSA), which was signed into law by congress on November 27, 2013.
DSCSA traceability requirements are being implemented gradually in multiple steps.
- Since 2015, healthcare organizations have been required to ensure that the prescription drugs that fall within the jurisdiction of the DSCSA are traceable at the lot level.
- Beginning on November 27, 2023, healthcare organizations must ensure that prescription drugs are electronically traceable at the package level — and this traceability must be interoperable with each of the other organizations in that drug’s supply chain.
The Drug Quality and Security Act is primarily responsible for ensuring interoperable traceability.
But what is traceability in DSCSA terms?
Most of the first phase of DSCSA requirements (the ones that have been in effect since 2015) will continue to apply after the second phase of requirements takes effect on November 27, 2023, but some of the phase one requirements will no longer apply after the phase two start date.
For example, after November 27, 2023, transfers of products are no longer required to be accompanied by the product’s full transaction history extending back to the original manufacturer.
The additional DSCSA requirements that will go into effect starting November 2023 highlight the need for reliable compliance management solutions at healthcare organizations.
As regulatory compliance requirements in the healthcare industry progress, organizations can leverage advancements in monitoring and reporting technology to keep pace.
Sonicu’s cloud-based monitoring and data logging solutions can make it possible for healthcare organizations to respond to almost any compliance audit related to temperature and environmental monitoring with only a few clicks.
Scores of pharmacies as well as drug distributors rely on Sonicu’s affordable temperature and environmental monitoring today to meet the stringest requirements enforced by the FDA, USDA and the Joint Commission.
You can learn more about how our software helps compliance professionals in these case studies:
Discount Drug Mart: Power Outage Protection + Compliance Automation
Problem: Transitioning from manual temperature logs and reactive power outage management
Solution: Adopting Sonicu's advanced system for seamless temperature monitoring and real-time power outage alerts across its 77 locations.
Hendricks Health: From Nutrition to the OR
Problem: The need to improve compliance and safety across various departments, starting from nutrition to the operating rooms.
Solution: Upgraded monitoring systems to enhance patient safety and streamline compliance across multiple facilities, including local YMCA rehabilitation facilities.
IU Health: Enterprise Monitoring
Problem: Challenges of using a locally based server and multiple monitoring solutions across departments.
Solution: Transitioned to a single, cloud-based solution with Sonicu to enhance monitoring capabilities across more than a dozen locations and the IU School of Medicine.
FDA DSCSA 2023
Under the authority of the FDA, DSCSA 2023 requirements consist of three main components:
- Interoperable exchange: For each transaction, organizations within a pharmaceutical supply chain must exchange Transactional Statements (TS) and Transactional Information (TI) in a manner that is electronic, secure, and interoperable with all other organizations in the supply chain.
- Interoperable tracing: For each unit sold, healthcare organizations must be able to trace the Transaction Statement and Transactional Information back to the manufacturer in a secure, electronic, interoperable manner, and must be able to produce these records upon request.
- Interoperable verification: For each unit sold, healthcare organizations must be able to verify the Product Identifier (which includes serial number, lot number, GTIN/NDC, and expiration date) in a secure, electronic, interoperable manner.
These are the main requirements that take effect under DSCSA law.
These requirements are intended to establish a fully interoperable system by which the legitimacy, safety and efficacy of prescription drugs can be electronically verified throughout the entire pharmaceutical supply chain.
However, there are also a few exemptions to DSCSA interoperability requirements.
Certain types of products and distribution cases are excluded from the law’s jurisdiction. Exemptions to DSCSA requirements include:
- Intracompany distribution of pharmaceutical products
- Product sample distribution
- Over-the-counter drug distribution
- Medical gas distribution
- Medical convenience kits
- Certain approved drugs for animals
- Blood and components for transfusion
DSCSA 2023 Timeline
DSCSA compliance requirements are being rolled out incrementally over a ten year period.
The Drug Quality and Security Act was enacted by the U.S. Congress in 2013 on November 27th. Title II of the DQSA, the Drug Supply Chain Security Act, first took effect several years ago in 2015.
However, there is still another phase left before DSCSA interoperability requirements are fully implemented.
On November 27, 2023, the second phase of Drug Supply Chain Security Act requirements will take effect.
The newer DSCSA 2023 requirements outline several different standards for the organizations that make up drug supply chains regarding interoperable exchange, verification, and traceability of Transactional Statements and Transactional Information.
DSCSA Timeline
Right now, the most important date for healthcare organizations to remember is November 27, 2023.
This is the date when the second phase of the Drug Supply Chain Security Act’s requirements will officially take effect.
However, there have been several other important steps that have taken place up until now as the DSCSA is gradually implemented.
- November 27, 2013: U.S. Congress enacts the Drug Supply Chain Security Act.
- January 1, 2015: Pharmaceutical manufacturers are required to print lot numbers on all prescription drug packaging.
- November 27, 2018: Pharmaceutical manufacturers are required to print expiration dates and unique serial numbers on all prescription drug packaging in both a human-readable format and in GS1 barcode format.
- November 27, 2019: Pharmaceutical wholesalers must verify the authenticity of all prescription drugs before reselling them.
- November 27, 2020: Medication dispensers must verify the authenticity of all prescription drugs before reselling them.
- November 27. 2023: Phase two of the DSCSA, including fully interoperable, unit-level traceability will be implemented throughout the entire prescription drug supply chain.
DSCSA 2023 Deadline
The deadline for the requirements established by phase two of the Drug Supply Chain Security Act is November 27, 2023.
This is the most important upcoming date to remember regarding DSCSA interoperability compliance.
Every organization throughout the whole prescription drug supply chain will need to achieve full DSCSA compliance by no later than this date.
For now, healthcare organizations are still only subject to the standards set forth by phase one of the Drug Supply Chain Security Act, which went into effect on January 1, 2015.
However, DSCSA requirements in 2023 will change when, on November 27th, phase two of the DSCSA’s requirements will go into full effect, mandating further expectations for organizations regarding electronic traceability and interoperability.
DSCSA 2023 Pharmacy Requirements
It’s essential for every organization in the pharmaceutical supply chain to understand the Drug Supply Chain Security Act pharmacy responsibilities that will be going into effect in late 2023.
Even though the deadline for complying with the second stage of DSCSA law has not yet arrived, it can be beneficial to plan ahead for DSCSA 2023 requirements rather than wait until the last minute and risk penalties for violating the new regulation.
One of the best ways to improve regulatory compliance throughout the pharmaceutical supply chain is to implement automated temperature and environmental monitoring solutions.
Using software tools to monitor and record temperature, humidity, air pressure, and more can reduce the risk of prescription medications being compromised due to unsafe storage conditions.
Automated monitoring and reporting tools can also greatly improve an organization’s ability to maintain compliance records and respond efficiently to audit requests.
Sonicu’s remote monitoring kits for healthcare and pharmaceutical organizations are some of the most effective solutions for boosting compliance-readiness and reducing risk of manual error.
Sonicu customers leverage the SoniCloud dashboard for convenient access to monitoring data at any time, from any location.
Sonicu’s reporting tools also make compliance reporting much easier by enabling organizations to respond to compliance audits with just a few easy steps.
Organizations can use software solutions for remote monitoring and data logging to help them accomplish compliance with DSCSA requirements in time for the deadline in November 2023.
You can learn more about how our software helps compliance professionals in these case studies:
Cryopoint: Mission-Critical Monitoring
Problem: Cryopoint faced calibration challenges with their legacy monitoring system and issues with a server-based system, putting critical samples at risk.
Solution: Transitioned to Sonicu's SoniCloud and its mobile-first solution, enhancing reliability in monitoring and easing operations.
Ohio University Innovation Center
Problem: Tech and tech-enabled startups at the Innovation Center needed reliable support systems for temperature and environmental monitoring due to the requirements of grant-funded projects.
Solution: Implement Sonicu for comprehensive monitoring of temperature, ambient humidity, and air pressure differential, ensuring necessary protection and compliance.
Hancock Regional Health: Enterprise Monitoring from Sound to Environmental
Problem: Disparate monitoring systems were taxing the staff with many manual processes, leading to inefficiencies and reduced asset protection.
Solution: CEO Steve Long initiated the adoption of a consolidated monitoring program for temperature, environmental, and sound, which automated compliance and reduced manual logging.
DSCSA Guidance
Every organization within the pharmaceutical supply chain in the United States is required to hold a license obtained from either the FDA or State Board of Pharmacy.
It is the ongoing responsibility of each pharmaceutical organization to verify that it does business only with organizations that are properly licensed.
DSCSA law also stipulates that in order to maintain DSCSA compliance, organizations in the pharmaceutical supply chain must include Transactional Statements and Transactional Information with all product shipments.
Transactional Statements (TS)
A Transactional Statement is a document that verifies the pharmaceutical organization selling the product:
- Is properly registered and authorized
- Received the product from an authorized and registered source
- Acknowledged any previous seller’s TS and TI
- Has not intentionally tampered with the transaction history
- Has not intentionally included any harmful or counterfeit product
Transactional Information (TI)
The Transactional Information included with a pharmaceutical shipment is a document that outlines the specifics of the transaction, including:
- The product name
- The strength and intended dosage of the product
- The date of the transaction
- The date of the shipment
- The product’s lot number
- National Drug Code
- Information about the container
- The number of containers
FDA DSCSA Guidance
It’s crucial for pharmaceutical organizations to understand the DSCSA timeline.
2023 will introduce new DSCSA interoperability requirements that must be met by all organizations in the prescription drug supply chain by no later than November 27, 2023.
DSCSA Summary
The first phase of the Drug Supply Chain Security Act, including certain FDA serialization guidance, took effect on January 1, 2015. DSCSA 2023 requirements will officially take effect beginning on November 27, 2023, and include requirements that primarily relate to the interoperable exchange, tracing, and verification of products throughout the pharmaceutical supply chain.
How IU Health
consolidated all of its pharmacy monitoring needs
into one cloud-based platform serving dozen of locations.
American-based Customer Support: Robust & Reliable High Touch Service
Software and technology is only as good as the people who stand behind it.
At Sonicu, that means our team of American-based customer success managers who are never more than a phone call away to help field and fix any service issues.
Our probes and sensors are placed in demanding frozen environments and our software literally sends billions bits of data monthly, meaning there’s always the potential for a hiccup on either the hardware or software.
We are committed to fielding every customer service request promptly and addressing our customer’s concerns promptly and professionally.
 “I like to say that every refrigerator or freezer is like a car in that they all behave a bit differently,
“I like to say that every refrigerator or freezer is like a car in that they all behave a bit differently,
and then every now and then you just get a bad boy who doesn’t want to perform as we need it to,”
Martha Rardin, Director, Nutrition and Dietetics, Hendricks Regional Hospital.
 “Sonicu has been a powerful tool to identify which units are behaving out of spec and get our team
“Sonicu has been a powerful tool to identify which units are behaving out of spec and get our team
to fix them before we have a serious issue.”
Tim Livesay, Director, Hancock Regional Hospital Pharmacy Director











Before Sonicu, we had to don bunny suits to check the status of our cleanrooms. Now we check our phones and know right away. The system saves us time and effort and helps us respond to environmental