FDA 483 Warning Letters
If you have received a 483 warning letter or you are looking for more information on how to permanently automate and fix compliance so you don't receive a letter from the FDA, then you've come to the right place.
Let us help you evaluate your needs!
- Safety: Alerts to protect asset
- Compliance: Automated reports
- Efficiency: Reduced Manual Logging
And what makes us different?
- Lifetime Warranty: Never buy hardware again!
- Unlimited Users: Scale across your entire org
- Connectivity Flexibility: Wi-Fi, Cellular or Data Hub
- Phone call alarms: Alerts won't get ignored
- Mobile App: 500 Freezers in your pocket
All American made and supported!
The Sonicu Difference
See What Customers Say About Sonicu
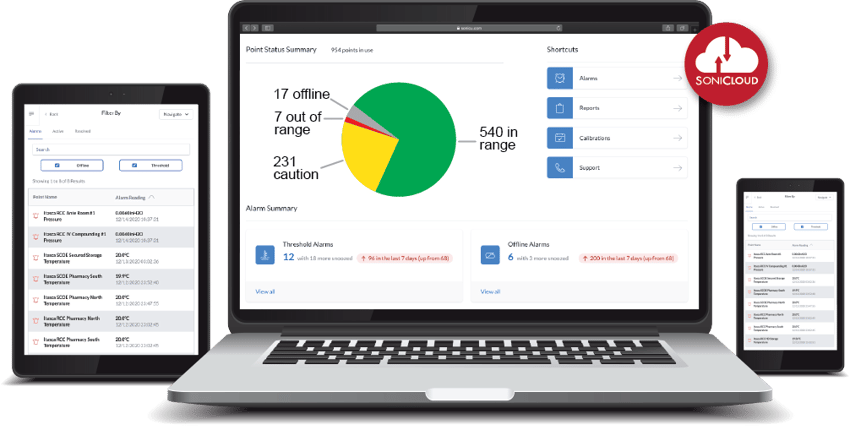
Asset Protection. Compliance Automation. And Reduced Manual Processes.
Sonicu serves thousands of professionals at hundreds of organizations across North America by improving how they monitor and manage their most sensitive assets and environments.
Professionals from healthcare, life science, laboratory and cold chain facility management turn to Sonicu to help them improve the way they do business.
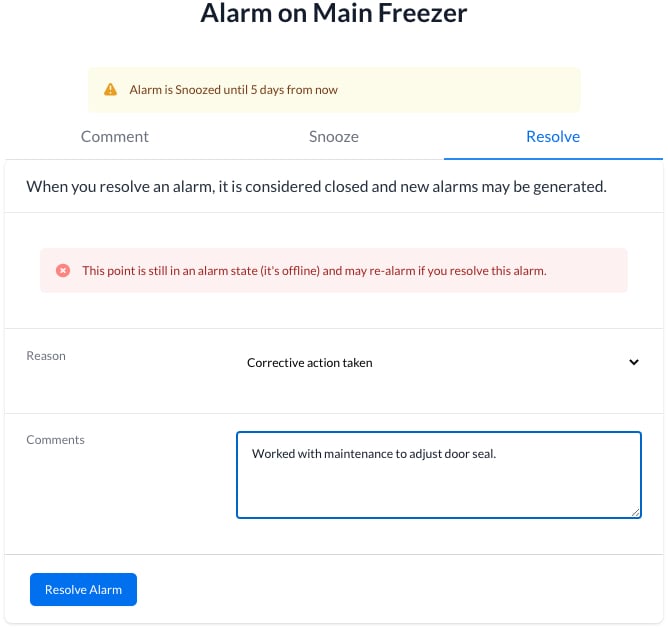
Worry Free Audits With 2 Click Compliance
FDA Inspection Warning Letters
Warning letters of any kind are rarely fun, but FDA 483 warning letters are especially disagreeable.
But what is an FDA inspection warning letter?
This is the letter issued by the Food and Drug Administration to inform the management of a company that they found conditions and/or practices within that company that could possibly be in violation of the Food Drug and Cosmetic (FD&C) Act and related regulations.
FDA 483 warning letters do not necessarily provide a complete list of potential violations present within a company. FDA investigators are expected to take note of the conditions they observed during that particular inspection.
The FDA investigators will communicate this FDA warning letter to the senior management of the company at the end of the routine inspection.
This letter is not just sent to the company by mail or email; investigators will meet with the management, read the letter aloud, and discuss the letter so there are no ambiguities or misunderstandings about the findings.
Bear in mind that the FDA observation warning letter does not automatically mean that a company is in direct violation of a regulation and that immediate action will be taken. It is not an instant indictment.
Instead, the FDA 483 inspection warning letter is a means by which the FDA can raise concerns to a company and the investigators will consider all possible evidence regarding the potential violation and the company’s response to that warning letter before deciding whether or not further action is required to ensure the safety of the public.
As such, an FDA 483 warning letter can serve as an opportunity for a company to address potential issues and ensure that it is in full compliance with what is expected within its industry.
FDA Warning Letter Database
If you want to know which companies have been issued FDA 483 warning letters, there is an entire database for it.
This database is located under the tap of “Compliance Actions and Activities, which in turn is housed under the “Inspection, Compliance, Enforcement, and Criminal Investigations” tab.
At the time of writing this article, there are 3,075 entries. This FDA warning letter database provides the following information to the public.
- The company’s name
- The issuing office (e.g. Center for Tobacco Products)
- The subject of the potential violation
- The date that the letter was issued
- The date that the record was posted to the database
The database also has columns dedicated to both response letters and closeout letters, though these spaces are not always filled in. A user can even export the findings.
What happens after an FDA warning letter depends on the nature of the potential violation and how the company responds to the letter’s findings.
When responding to FDA warning letters, pharmaceutical companies, for example, need to carefully review the findings, whether the warning letters pointed to the use of unapproved drugs, questionable storing conditions, or any other cause for concern.
Given that the general public has access to this information, it is in the best interest of any company to see that all concerns are addressed and conditions possibly rectified so as not to sow doubt among its customers.
FDA 483 Database Search
Not only is the information available for FDA 483 warning letters in a long list of FDA inspection reports, but one can use this database to search specific FDA 483 warning letters through a variety of filters.
Within the FDA 483 database search, there is a general search tab, but in addition to that, you can search by the following identifiers.
- issuing office
- Letter issue date
- Letters with respond or closeout letters
- Posted date
- Year of incident
There is also an email subscription option where users can enter their email address and then receive updates on FDA inspectors, FDA inspection classification definitions and other related topics.
As mentioned above, this database is accessible to the public, so companies should move swiftly in tackling any issues that the FDA 483 warning letter may include.
Types Of FDA Warning Letters
There are multiple types of FDA warning letters. The specific type of letter that a company receives will depend on the nature of the issues noted in the observation.
These differing letters highlight different possible FDA violation examples. Here is a list of the various types.
- General FDA Warning Letters
- Tobacco Retail Warning Letters
- Drug Marketing and Advertising Warning Letters
- Warning Letter Close-Out Letter Program
General FDA warning letters are the most common type, being issued when an FDA investigator observes what could be a substantial violation of FDA regulations. These warnings typically mean that a company needs to address the issues stated in the letter to avoid FDA enforcement actions.
Tobacco Retail warning letters are specifically designed for tobacco sellers to ensure they are in compliance with Federal Food, Drug, and Cosmetic Act. The Drug Marketing and Advertising warning letters are geared toward the compliance of companies in providing only approval and legal drugs to their customers.
In regard to FDA warning letters, pharmaceutical companies may possibly be one of the drug marketing and advertising type. A Close-Out letter is a letter that the FDA issues after corrective actions have been taken to resolve the issues of the original warning letter.
What happens after FDA warning letter?
The company is given the opportunity to respond and rectify the issue or provide evidence that they are not, in fact, in violation of any regulations. No company is above inspection.
FDA 483 Observations
FDA 483 observations are the notice that the FDA gives to companies before FDA 483 warning letters. An observation may not be a full inventory of a company’s compliance with the complete list of FDA 483 requirements, but it contains the observations that FDA inspectors took note of during a routine inspection.
If an observation escalates to an FDA warning letter, pharmaceutical companies and other similar businesses are required to take corrective action.
Observations could be issued for a number of FDA violation examples. The cause for the most common FDA 483 observations include the following:
- Procedures not completely followed
- Poor investigations of discrepancies or failures
- Lack of written procedures
- Concerns for data integrity
- Cleaning, sanitizing, and maintenance
- Monitoring of environment
When it came to FDA warning letters, 2015 was a busy year as the FDA issued no less than 47 warning letters.
FDA Form 483
FDA form 483 is the form by which the FDA documents and communicates concerns that are discovered during inspections.
An FDA 483 example looks very similar to a tax form where there are several boxes to fill out basic administrative information for the company with larger boxes where the investigators may go into detail about their observations.
These forms can be found in an FDA 483 database on their official website.
FDA Form 483 Response Time
After the issuance of FDA warning letters, pharmaceutical companies and other entities are required to respond to remedy the observations recorded in the letter.
But what is the FDA form 483 response time?
The moment that the FDA issues the observation to the company, the recipient has exactly 15 days to respond. Therefore, an FDA 483 warning letter response is tracked on quite a tight schedule, depending on the number of violations that were found and need addressing.
Remember that you should only craft an FDA form 483 observation response when you are calm and have shaken off the initial shock and anger.
The FDA is not designed to put you out of business; it simply exists so that all businesses operate on the same level to ensure the safety of their employees and customers.
American-based Customer Support: Robust & Reliable High Touch Service
Software and technology is only as good as the people who stand behind it.
At Sonicu, that means our team of American-based customer success managers who are never more than a phone call away to help field and fix any service issues.
Our probes and sensors are placed in demanding frozen environments and our software literally sends billions bits of data monthly, meaning there’s alway the potential for a hiccup on either the hardware or software.
We are committed to fielding every customer service request promptly and addressing our customer’s concerns promptly and professionally.
 “I like to say that every refrigerator or freezer is like a car in that they all behave a bit differently,
“I like to say that every refrigerator or freezer is like a car in that they all behave a bit differently,
and then every now and then you just get a bad boy who doesn’t want to perform as we need it to,”
Martha Rardin, Director, Nutrition and Dietetics, Hendricks Regional Hospital.
 “Sonicu has been a powerful tool to identify which units are behaving out of spec and get our team
“Sonicu has been a powerful tool to identify which units are behaving out of spec and get our team
to fix them before we have a serious issue.”
Tim Livesay, Director, Hancock Regional Hospital Pharmacy Director