FDA DSCSA Guidance
If you’re looking for a cloud-based monitoring solution that eliminates manual logging, improves FDA DSCSA compliance readiness and protects all your clean rooms and compounding rooms, you’ve arrived at the right place.
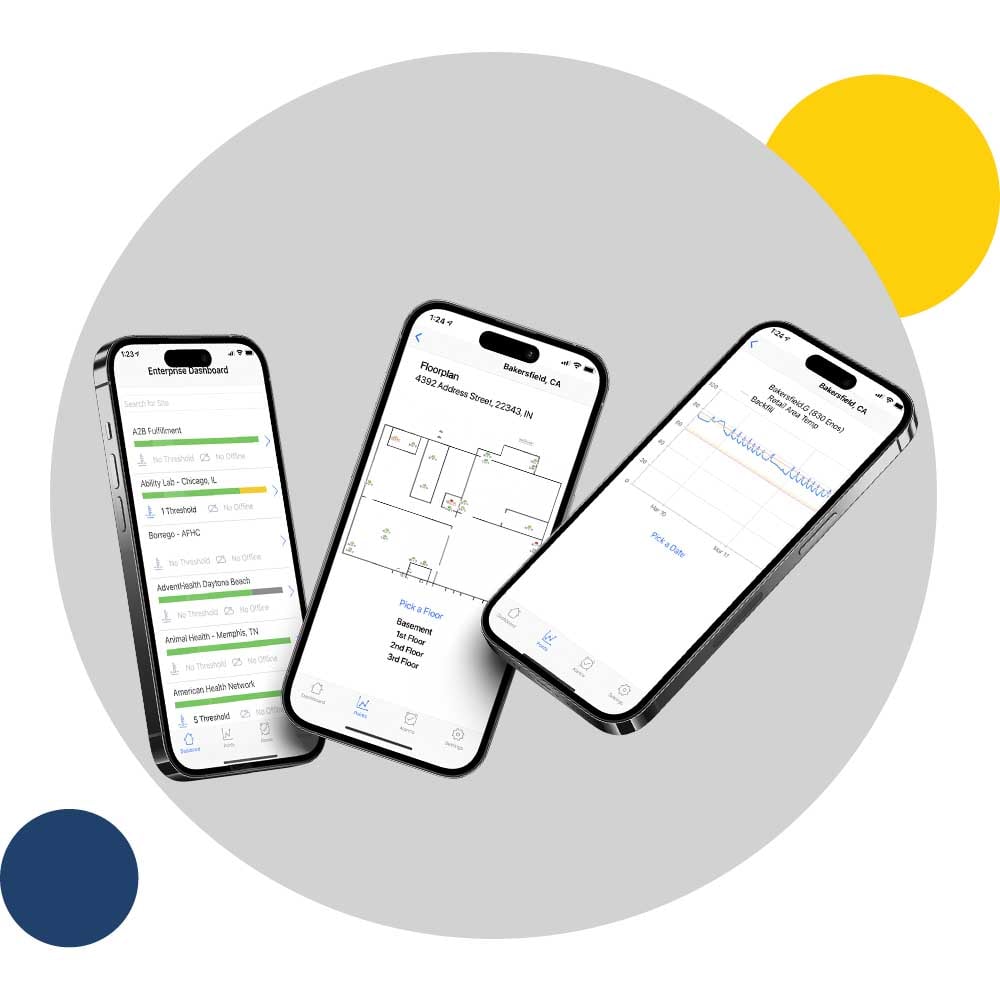
Let us help you evaluate your needs!
- Safety: Alerts via text, email, push notifications and phone calls to protect your precious assets
- Compliance: Automated compliance reports
- Efficiency: Reduced Manual Logging and time spent on reports
And what makes us different?
- Lifetime Warranty: Never buy hardware again!
- Unlimited Users: Scale across your entire organization
- Connectivity Flexibility: Wi-Fi, Cellular or Data Hub
- Phone call alarms: Alerts won't get ignored
- Mobile App: 500 Freezers in your pocket
- Facility monitoring: Simple to add water leak, door open, occupancy, and even IAQ monitoring
Engineered in Indiana with U.S.-based support.
Sonicu costs are considerably more reasonable than our historic system, as well as other competitors on the market. The equipment is robust but simple to learn and utilize.

Having safe and secure storage provides reassurance that any future family building efforts will be protected. Sonicu gave us the ability to more easily put our head on the pillows and sleep easier at night knowing we had invested in a strong monitoring system.”

See What Customers Say About Sonicu
Asset Protection. Compliance Automation. And Reduced Manual Processes.
Sonicu serves thousands of professionals at hundreds of organizations across North America by improving how they monitor and manage their most sensitive assets and environments.
Professionals from healthcare, life science, laboratory and cold chain facility management turn to Sonicu to help them improve the way they do business.

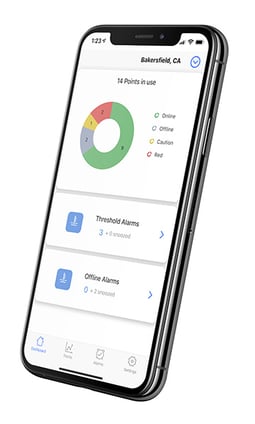
- Real-Time Monitoring: The sensors collect temperature data and transmit it wirelessly to Sonicloud - our cloud-based platform.
- Operational Efficiency: Virtually eliminate the need for tedious and costly manual logging
- Compliance Automation: Respond to virtually any regulatory audit or inspection in a few clicks with our reports section
- Asset Protection: Detect and respond to any temperature excursion that can threaten virtually anything perishable: food, drugs, vaccines, research, etc.

How IU Health
consolidated all of its pharmacy monitoring needs
into one cloud-based platform serving dozen of locations.
FDA DSCSA Guidance
The pharmaceutical industry is one of the most heavily regulated industries in the world.
Remaining compliant with the government's many laws and regulations can sometimes be a daunting task, especially if you don’t have the right tools at your disposal. One of these laws that you may have heard of is the FDA’s Drug Supply Chain Security Act (DSCSA).
What is the drug supply chain security act?
Simply put, it is a new regulation designed to secure and enhance the overall supply chain carrying medicine from the manufacturers to the patients. In this article, we’re going to go over some of the details and requirements placed by this new law to help give you a better idea of how to remain safe and compliant.
For example, we’ll discuss DSCSA serialization and the obligations associated with the DSCSA pharmacy requirements. For further information, there is a plethora of FDA DSCSA guidance available on the FDA website.
This article will also explore the role temperature and environmental monitoring can play in the safe and profitable manufacturing, storage and delivery of pharmaceutical drugs with a special emphasis on the experience Sonicu has in this field.
Today Sonicu supports dozens of hospital-based pharmacies as well as scores of regional pharmacy chains and even a handful of regional distributors with our simple and affordable temperature and environmental monitoring solutions.
The DSCSA is a complex and ambitious law, and it will take several years to fully implement. However, the act has the potential to significantly improve the security of the pharmaceutical supply chain in the United States.
Here are some of the reasons why the U.S. government created the DSCSA:
- To protect patients from counterfeit and diverted prescription drugs.
- To improve the efficiency of the pharmaceutical supply chain.
- To reduce the costs of drug recalls.
- To deter drug theft and diversion.
- To strengthen the U.S. pharmaceutical industry's competitiveness in the global market.
The DSCSA is a significant step forward in the fight against counterfeit and diverted prescription drugs. However, it is important to note that the act is not a silver bullet.
There are still many challenges to overcome, and it will take a concerted effort from all stakeholders to ensure the success of the DSCSA.
The DSCSA places requirements on manufacturers, trading partners, and pharmacies (which are called dispensaries in the law).
These requirements are designed to secure the drug supply of our nation. At a high level, the main thrust of the legislation is that products must be tracked and traced at the package level throughout the supply chain with electronic systems.
This tracking will enable the FDA to quickly identify any supply chain problems and address them before any patients are negatively impacted. Some of the legislation first came into force in early 2015, and the rest of it has continued to be phased in gradually. The final aspects of the law will come into full effect in 2023.
The DSCSA is only one of many legal regulations placed on drug manufacturers and pharmaceutical organizations. Others place requirements on the safe transport and distribution of drugs as they move through the supply chain.
Environmental threats to the safety and quality of pharmaceutical products are one of the threats to the safety and quality of pharmaceutical products.
When transporting drugs and vaccines, conditions such as temperature and humidity are often highly critical and must be carefully monitored.
Historically, this has meant hundreds of thousands of hours spent manually tracking the temperature of products, logging those temperatures, and reporting on them.
However, this process is highly inefficient and has repeatedly resulted in failures causing the loss of products and interrupting the supply chain.
That’s why Sonicu has developed the solution – a cloud-based, wireless monitoring system. Our sensors can monitor a variety of environmental conditions and can be one of the best ways to remain compliant with transport and distribution requirements.
Today, Sonicu offers a battery-powered, self-install temperature and humidity sensor with NIST certifications that is ideal for a warehouse application and can even be placed in mobile transport coolers to capture temperature and environmental conditions while drugs are in final transport to a retail location, allowing pharmaceutical distribtutors to have real-time and historical access to regulatory data from the moment they receive drugs from the manufacturer to the point of releasing them to a retail outlet.
You can learn more about how our software helps compliance professionals in these case studies:
Problem: Pharmacy Suffered Too Much Humidity In New Wing, impacting Compounding Pharmacy
Solution: Affordable Humidity Monitoring that Delivered Powerful data to prompt contractors to fix improperly sized air handler
Problem: Dining department struck with regulatory violations
Solution: Enterprise-wide monitoring that automates regulatory compliance across all departments
Problem: Release of lead particles in battery projects
Solution: Mobile, affordable air pressure monitoring solution
DSCSA Manufacturer Requirements
According to the DSCSA guidance, one of the most important DSCSA manufacturer requirements is serialization.
As of November 2020, all of the main operators in the pharmaceutical supply chain, including manufacturers, must generate, authenticate, and verify serial numbers for all packages in the supply chain.
These serial numbers are a critical component of the overall goal of ensuring that transaction information is recorded at every stage of a product’s journey through the supply chain.
If a product is malformed or tampered with, these numbers will enable the FDA and other trading partners to rapidly investigate the source of the problem and address it as quickly as possible.
Another question that often comes up when studying this new law is, what is the DSCSA definition of a return under the DSCSA?
Returns are yet another part of the supply chain that must be monitored to ensure that only high-quality, untampered products are allowed back into the supply chain.
The DSCSA requires that wholesalers must verify saleable returns before they can be resold. Each and every drug must be declared safe and legitimate in order for it to be added back into the overall drug supply chain.
Drugs will be deemed safe to return to sale under the terms of the DSCSA if they meet the following criteria:
- They must be genuine, meaning that they must be manufactured by an authorized manufacturer and have not been tampered with.
- They must be intact, meaning that they must not have been damaged or altered in any way that could affect their safety or efficacy.
- They must be accompanied by a valid pedigree, which is a document that tracks the movement of the drug through the supply chain.
- They must be approved by the FDA for sale in the United States.
If a drug meets all of these criteria, it wil be deemed safe to return to sale. However, the FDA may still require additional testing or verification before the drug can be released back to the market.
The following are the steps that will be taken to determine if a drug is safe to return to sale under the terms of the DSCSA:
- The drug will be inspected to verify its authenticity and integrity
- The drug’s pedigree will be reviewed to ensure that it is accurate and complete
- The drug may be tested to confirm its safety and efficacy
- The FDA may require additional information or documentation before making a final decision
The FDA will issue will issue a clearance letter when the drug is cleared to be released.
Throughout this time-consuming administrative process, the need to protect the drugs from loss due to temperature and environmental threats will remain.
You can learn more about how our software helps compliance professionals in these case studies:
Problem: Data loggers that were not on the cloud and cloud-based solution that only connected via wi-fi
Solution: Cloud-based solution with connectivity in wi-fi AND cellular for maximum flexibility and compliance readiness
Problem: The aging walk-in cooler with a faulty electrical system caused system failure and a loss of $5,000 in food
Solution: Simple and affordable temperature monitoring solution that protects costly food and automates compliance.

FDA DSCSA
As shown in the FDA DSCSA guidance, the DSCSA penalties for violation are severe.
Failure to comply with this legislation can result in fines, suspension, or revocation of license and can even lead to imprisonment or civil penalties.
This is why it is so important to ensure that you fully understand the FDA DSCSA obligations and have complied with them. Looking ahead, you may be wondering what the DSCSA 2023 timeline looks like.
In 2023, the final requirements of the law are put into force.
These consist of two components.
Firstly, full interoperable electronic unit-level traceability systems must be implemented.
Then, verification of saleable returns must also be put into place. However, don’t wait until 2023 to ensure that you are implementing these regulations. You might be too late.
One of the key recommendations by the FDA when it comes to complying with this new regulation is that you rely on the EPCIS standard. The EPCIS standard is the electronic product code information services standard and is widely used for maintaining the integrity of modern supply chains.
The FDA recommends using this standard to provide and maintain the data associated with transaction information and statements. Overall, the guidance encourages drug manufacturers to work with trading partners and dispensaries to achieve an overall more secure supply chain.
While there are no specific requirements associated with temperature and environmental monitoring, there is serious risk to drugs safety and efficacy if they fall outside of these prescribed ranges during shipment and/or storage.
Moreover, there are FDA and CDC recommendations tied to temperature and environmental standards that will impact the same group of organizations facing the DSCSA requirements.
Sonicu is a leading provider of simple and affordable monitoring solutions that make it easy for vrituallly any organization to deploy a solution without significant technical and IT investment.
Drug Supply Chain Security Act Pharmacy Responsibilities
The law is quite clear when it comes to understanding the drug supply chain security act pharmacy responsibilities. If you were to look at a drug supply chain security act summary, you’d see that it is vital that you, as a pharmacy, check to ensure that your trading partners are licensed or registered (depending on their role in the supply chain).
Most of the requirements in the original law have now come into effect according to the drug supply chain security act timeline. This includes the requirement for pharmacies to only accept prescription drugs that are accompanied by three pieces of product tracking information.
These are:
- the transaction info
- transaction history
- and transaction statement
Furthermore, these three pieces of information must be stored and recorded in either paper or electronic form for at least six years.
Finally, all of this documentation must be generated and provided to any trading partner to whom you may sell the prescription drugs.
In addition to these requirements for tracking and tracing information, the law also requires pharmacies to have a process for investigating and handling illegitimate prescription drugs.
This process must include three elements.
- The pharmacy must quarantine and investigate any suspect prescription drugs to check if they are illegitimate.
- If they are, the pharmacy must also have a process in place to take specific steps to prevent patients from receiving those illegitimate drugs.
- Finally, the pharmacy is required to notify the FDA.

DSCSA Compliance
In order to remain in DSCSA compliance, your organization must comply with the new requirements of the drug supply chain security act 2023.
For more information on the DSCSA requirements, you could download a drug supply chain security act PDF from the internet. This will help to ensure that you never miss a DSCSA deadline.
Other regulations outside of the DSCSA from the FDA place many restrictions on how drugs are stored and transported to their locations. Remaining compliant may be cumbersome, but automated remote monitoring solutions designed specifically for pharmaceutical distribution can make things much easier.
Sonciu sensors are designed to deploy quickly, providing superior asset protection straight out of the box.
With a sensor package from Sonicu, you can eliminate manual data logging and simplify regulatory compliance, enabling you to ensure the safety of your distribution chain.
Our platform is cloud-based, allowing you to access detailed data on your products from anywhere and at any time. Our sensors provide monitoring for all kinds of environmental conditions, including:
- Temperature
- Humidity
- Air Pressure Differential
- Sound
- Pressure
- ULT and Cryogenic
All of our monitoring solutions are fully compliant with all state and federal regulatory requirements. With effortless automation, you can say goodbye to all the manual monitoring and logging.
Furthermore, our platform is designed to be totally secure and is independent of your existing IT infrastructure. This means that even if your systems are breached, our monitoring platform will continue to operate and alert you to any changes that might impact the safety of your products.
DSCSA Exemptions
The guidelines from the FDA state that there are DSCSA exemptions.
The primary exemption for tracking and tracing this transaction information is for any products that are being distributed for emergency medical reasons. Exemptions to DSCSA requirements include any drugs being produced pursuant to a public health emergency declaration.
Exemptions to this regulation are limited, and you should check to ensure that your products are truly excluded from the requirements stated.
The DSCSA governs pharmaceutical distribution.
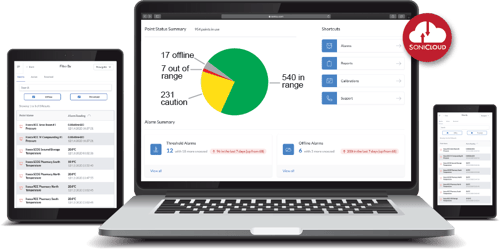
One of the best tools for maintaining the safe distribution of your products is the Sonicu Monitoring Dashboard. Each of the sensors we provide is automatically connected to this cloud-based dashboard, providing a scalable platform that makes it easy to view all your monitoring at any time.
Our dashboard is accessible on mobile and is hosted on AWS for best-in-class security and reliability. This tool makes it easy to get a comprehensive view of what is happening with your products in real-time.
DSCSA Timeline
The DSCSA timeline has been a critical component of the DSCSA law for several years. The first phase of the law came into force in 2015.
Three years later, DSCSA serialization requirements were first placed on pharmaceutical manufacturers and re-packagers.
Then in 2019 and 2020, additional requirements were put into force as part of this law. Finally, by 2023, each component of the supply chain will need to be compliant with every element of the DSCSA.
In order to meet these deadlines, organizations in the pharmaceutical industry must create and execute plans for providing these tracking and tracing features.
Ultimately, the goal of the DSCSA is to secure the United State’s drug supply chain. However, those products are at risk if those drugs and vaccines are not kept in the correct environmental conditions. Sonicu provides a different level of monitoring from the competition.
With advanced alarming, SMART reporting, the safest VPN, and easy recalibration features, you can always have peace of mind that your products are secure, wherever they may be.
American-based Customer Support: Robust & Reliable High Touch Service
Experience Unparalleled Service with Sonicu's Dedicated Team
At Sonicu, we understand that the true value of software and technology lies in the people who stand behind them.
Our probes and sensors are purposefully designed to withstand the harshest frozen environments, working tirelessly to collect and transmit billions of bits of data every month.
We recognize that even with the most advanced technology, occasional hiccups can occur, whether it's on the hardware or software side. But rest assured, our commitment to you is unwavering.
That's why we take pride in our team of dedicated American-based customer success managers, always ready to assist you with any service issues. With Sonicu, help is just a phone call away, ensuring a seamless experience for our valued customers.
Our customers are highly educated and protect highly valuable assets with our solutions. We boast more than 95 percent customer retention thanks to our reliable hardware, intuitive software and robust customer support.
At Sonicu, we prioritize customer satisfaction and swiftly address any support request that comes our way. Our responsive team is dedicated to resolving issues promptly and getting our customers back online without delay.
We are committed to fielding every customer service request promptly and getting our customers online rapidly.
 “I like to say that every refrigerator or freezer is like a car in that they all behave a bit differently,
“I like to say that every refrigerator or freezer is like a car in that they all behave a bit differently,
and then every now and then you just get a bad boy who doesn’t want to perform as we need it to,”
Martha Rardin, Director, Nutrition and Dietetics, Hendricks Regional Hospital.
 “Sonicu has been a powerful tool to identify which units are behaving out of spec and get our team
“Sonicu has been a powerful tool to identify which units are behaving out of spec and get our team
to fix them before we have a serious issue.”
Tim Livesay, Director, Hancock Regional Hospital Pharmacy Director











Cost competitiveness, great customer service, great control over the monitoring system, and low maintenance. You can't beat that.